Finding the Vaccine: The Benefits and Risks of Speeding Vaccine Development
Finding the Vaccine: The Benefits and Risks of Speeding Vaccine Development
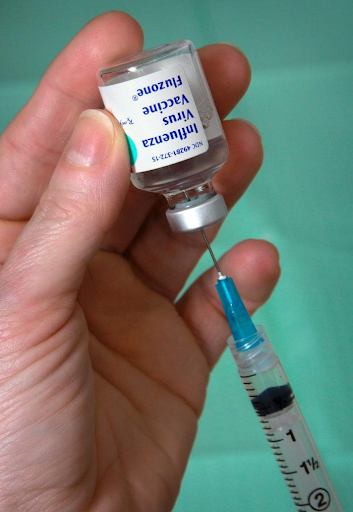
As the death toll from the COVID-19 pandemic rises, so has demand for vaccines. Scientists have tried to meet this demand by speeding up the process of creating and approving a vaccine. Typically, it would take 12-18+ months for a vaccine to make it through testing and development. The Ebola vaccine wasn’t widely distributed until early 2018, over three years after the initial outbreak. Thankfully, fundraising and public support have made it possible for labs around the world to speed up the process. However, some worry that foregoing some of the tests a vaccine would usually need before approval may foster a distrust of vaccines that the U.S. health system cannot afford right now.
The process by which vaccines are tested and approved for distribution relies on a few key factors. First, it is important to understand what makes COVID-19 special. Crucially, researchers had a head start on the COVID-19 vaccine due to previous research on similar diseases. Coronaviruses are a large classification of viruses, including SARS and some strains of the common cold. They have S proteins on their surface (the infamous spikes that create a “corona,” or crown, around the germ), which attach the virus to human cells and allow it to spread.
Simply put, vaccines introduce a non-viable form of a germ into a patient, so that the patient’s immune system produces antibodies against the disease. If the vaccine is effective, the presence of antibodies makes the patient immune. According to the Mayo Clinic, there are three main kinds of vaccines that researchers can choose from to combat COVID-19, each posing its own challenges. A live vaccine contains a weakened form of the virus but may still be dangerous for patients with weakened immune systems. An inactive vaccine contains an inactive “dead” form of the virus, which may be safer than the live vaccine but less effective. Some patients may require multiple doses of an inactive vaccine for it to work. Lastly, there are genetically engineered vaccines which contain RNA/DNA with instructions to copy an S protein’s structure, mimicking the structure of a coronavirus. The problem with this approach is that no genetically engineered vaccine has ever been licensed for human use, so the risks aren’t fully understood.
Regardless, the promising idea of genetically engineered vaccines is taking the scientific and medical communities by storm. Pulmonary disease specialist and Latin parent Dr. Kevin Kovitz said, “From a scientific perspective, it is very exciting to see completely new vaccine technologies, i..e. the RNA-based vaccines, emerging. Decades of basic science research seem to be paying off and facilitating more rapid development of practical applications of benefit to the general public.” Vaccinologists have learned so much over the past months, thanks in part to increasing funding for vaccine development. Unfortunately, it is still uncertain whether they have learned enough to make a safe and effective vaccine in less than a year.
The typical vaccine trial timeline starts with 3-6 months of animal testing before moving on to three phases of human testing. The three phases of testing on human patients include clinical trials to evaluate safety, then trials with hundreds of people to evaluate formulation and dosages, and, finally, another safety and efficacy trial where the vaccine is tested on thousands of people. After that, if all goes well, the vaccine is approved and distributed to the masses. Amy Merrell, an Upper School biology teacher, expressed concern with an expedited vaccine approval. “If we’re trying to speed this up, we may not see some of the long-term side effects because we didn’t do the studies long enough,” she said. “It’s not that increasing the speed that we develop the vaccine is going to increase the side effects. It’s just that we may skip some.”
Dr. Kovitz shared a similar concern. “I am hopeful that the sped-up process leads to a successful vaccine. However, the speed must not lead to skipped steps. Time is needed to establish efficacy and a tolerable rate of complications.”
A big concern surrounding the COVID vaccine is that, because the vaccine might be approved and distributed so quickly, people who are already skeptical of vaccination might refrain from getting it. “There is already too much vaccine hesitancy in society,” Dr. Kovitz said. “If political pressure, real or imagined, impacts the process, fewer will use even a vaccine that is shown to be effective.” Politics are increasingly tied up in medicine, and it may further affect the testing timeline.
With so many unknowns and interfering voices, these uncertain circumstances become all the more uncertain. Any prediction is questionable at best, and planning for the future is near impossible. “If the process truly sticks to science and not political pressure, the predictions should be reliable,” Dr. Kovitz said. “The caveat is that some facts, positive and negative, may come out over time. But, that is the reality of any new drug or technology. To paraphrase Donald Rumsfeld, George W. Bush’s Defense Secretary, there are known knowns, known unknowns, unknown unknowns, etc.”

Angela Gil (’21) is a senior at Latin and is honored to be serving as The Forum’s Features Editor. She has written for The Forum...